Cusabio N-terminal 6xHis-Flag-tagged Recombinant
FLAG system
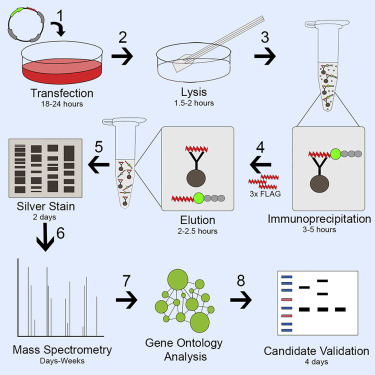
The isolation and analysis of protein complexes remain a great technical challenge in functional proteomics research. Co-immunoprecipitation is the most widely used method for the isolation of protein complexes. The TAP (tandem affinity purification) methodology incorporates tandemly linked affinity tags onto a target protein to extract and isolate endogenous interacting proteins. Protein complexes isolated by TAP have much higher purity compared to single purification.
FLAG System Overview
The FLAG tandem epitope system consists of small epitope tags that are not derived from eukaryotes. These features minimize interference with protein functions and provide superior specificity. The hydrophilic character of the FLAG peptide increases the likelihood that the dual epitope will be located on the surface of the fusion protein where it is accessible for antibody-antigen interaction.
Other TAP systems, including combinations of GSTs, clamodulin, nickel-binding proteins, streptavidin, and Protein A, have suffered from several limitations, such as interference with complex assembly or protein function due to relatively large TAP tags (20 KD And older); high rate of contamination with endogenous non-target proteins; and requirement of TEV protease treatment for elution which adds additional bacterial contaminants.
Key features of the FLAG system
- Affinity resins have high specificity and remove fewer contaminants compared to existing systems.
- The TAP tag does not sterically affect the interactions of the target proteins.
- No need to elute with protease
- Efficient and gentle elution for the first immunoprecipitation and flexible elution strategies for the second.
What is the FLAG tag?
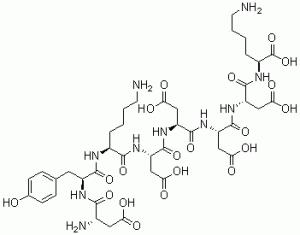
N-terminal 6xHis-Flag-tagged Recombinant, or FLAG octapeptide, or FLAG epitope, is the first epitope tag designed for fusion proteins and is the only patented tag. The molecular weight of the DYKDDDDK tag (FLAG tag) is 1012 Da. The multiple polyanionic amino acids in the FLAG tag are less likely to affect the activity of a target protein. The FLAG tag allows for highly specific dropdown menus that contain a low non-specific background.
A FLAG-tag can be used in many different assays that require recognition by an antibody. If there is no antibody against a given protein, adding a FLAG tag to a protein allows the protein to be studied with an antibody against the FLAG sequence.
Why use a FLAG tag for protein expression and production?
The addition of a FLAG tag to the N- or C-terminus of a protein allows rapid purification of the overexpressed recombinant protein by use of a FLAG tag-specific monoclonal antibody conjugated to agarose beads. Previously, commercial FLAG-tag affinity purification resin was very expensive and could only be used a couple of times. After many years of development, Sino Biological has developed an excellent FLAG tag-specific monoclonal antibody that can be used for various detection applications, as well as conjugated with agarose for affinity purification.
The improved FLAG-tag affinity resin is affordable and can tolerate low pH elution, which increases the shelf life of the resin. Furthermore, FLAG tag-specific antibodies can be used to detect the expression level of the tagged protein in cell culture, by ELISA and western blot, etc.
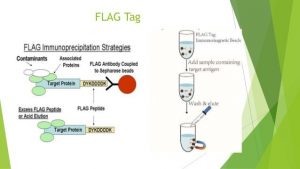
How to purify FLAG-tagged proteins?
FLAG-tagged recombinant protein can be affinity purified directly from cell culture lysate or supernatant. The FLAG-tagged protein is bound to the conjugated FLAG tag-specific monoclonal antibody on an agarose gel. After washing away residual impurities, the bound FLAG-tagged proteins can be eluted from the affinity column by a high concentration of the FLAG-tagged peptide or by a low pH buffer. For more information on the use of FLAG-tag affinity purification resin, see FLAG-tag Affinity Resin.
How do I remove the FLAG tag after purification?
In some applications, it is desirable to remove the FLAG tag, for example for protein crystallization. To allow cleavage of the FLAG tag, it is necessary to design a protease cleavage site between the tag and the protein. An EK cleavage site behind the FLAG tag (FLAG-EK site protein structure) may allow complete removal of the FLAG tag and cleavage site, leaving no additional amino acids after specific cleavage of the FLAG tag. For more information on cleavage site and tag removal by EK and HRV-3C protease, see Enterokinase (EK), HRV-3C (Human Rhinovirus Protease).
In conclusion, the FLAG tag shows all the advantages and disadvantages of immunoaffinity purification. Although highly selective, binding capacities are low, making scaling up an expensive undertaking.
